Embark on a journey into the fascinating realm of chemical reactions with our comprehensive synthesis and decomposition reaction worksheet. This valuable resource delves into the fundamental concepts, applications, and practical demonstrations of these essential processes.
Through engaging explanations and step-by-step guidance, you will gain a thorough understanding of synthesis reactions, where elements or compounds combine to form new substances, and decomposition reactions, where compounds break down into simpler components.
Types of Synthesis and Decomposition Reactions: Synthesis And Decomposition Reaction Worksheet
Synthesis reactions are chemical reactions in which two or more substances combine to form a single product. They are typically represented by the following equation:
A + B → AB
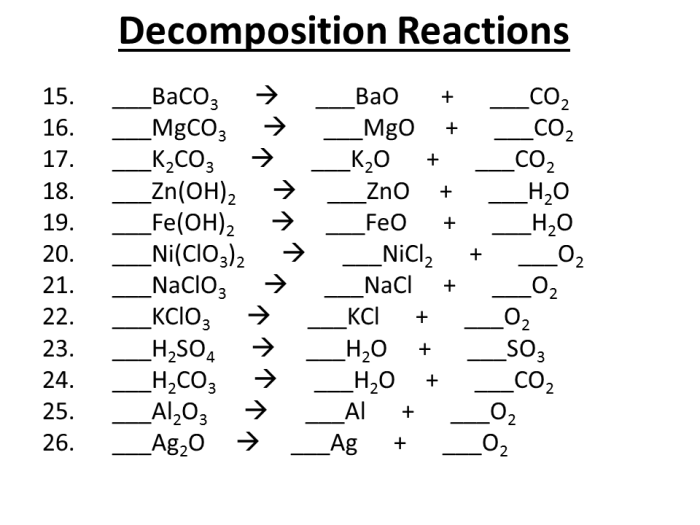
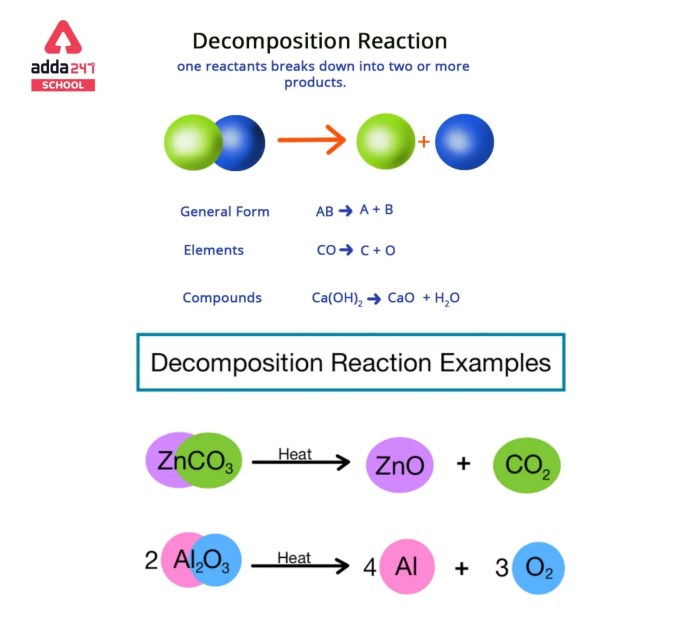
Decomposition reactions, on the other hand, are chemical reactions in which a single compound breaks down into two or more simpler substances. They are typically represented by the following equation:
AB → A + B
Examples of Synthesis Reactions, Synthesis and decomposition reaction worksheet
- The reaction of hydrogen and oxygen to form water: 2H 2+ O 2→ 2H 2O
- The reaction of sodium and chlorine to form sodium chloride: 2Na + Cl 2→ 2NaCl
- The reaction of iron and sulfur to form iron sulfide: Fe + S → FeS
Examples of Decomposition Reactions
- The decomposition of water into hydrogen and oxygen: 2H 2O → 2H 2+ O 2
- The decomposition of sodium chloride into sodium and chlorine: 2NaCl → 2Na + Cl 2
- The decomposition of iron sulfide into iron and sulfur: FeS → Fe + S
General Inquiries
What is the difference between a synthesis and a decomposition reaction?
In a synthesis reaction, two or more substances combine to form a new, more complex substance. In a decomposition reaction, a single substance breaks down into two or more simpler substances.
How do I balance chemical equations?
To balance chemical equations, adjust the coefficients in front of each reactant and product to ensure that the number of atoms of each element is the same on both sides of the equation.
What are some examples of industrial applications of synthesis reactions?
Synthesis reactions are used in the production of fertilizers, plastics, pharmaceuticals, and fuels.
What are some examples of environmental applications of decomposition reactions?
Decomposition reactions are used in processes such as composting, water purification, and the breakdown of pollutants.